.png?width=540&height=480&name=Q%20%26%20A%20(6).png) Pacific Star Labs Cannabis Chemistry Guide
Pacific Star Labs Cannabis Chemistry Guide
Start your chemistry journey with the Cannabis Chemistry Guide, your source for everything chemistry.
| Chapter 1 | Analytical Chemistry |
| 1.1 | Types of Analytes |
| 1.1 | Calibration |
| 1.3 | Instrumentation & Methods |
| 1.4 | Data Reporting |
| 1.5 | Method Development & Validation |
| 1.6 | Proficiency Testing |
| 1.7 | Instrument Calibration |
| 1.8 | Quality Assurance (QA) |
| 1.9 | Concentration |
| Chapter 2 | Cannabis Products |
| 2.1 | Consumption Pathways |
| 2.2 | Cannabinoids & Terpenes |
| 2.3 | Flower |
| 2.4 | Rosin |
| 2.5 | Hash |
| 2.6 | Extract & Distillate |
| 2.7 | Tincture |
| 2.8 | Transdermal Patch |
| 2.9 | Infused Products |
| Chapter 3 | Product Development |
| 3.1 | Proof of Concept |
| 3.2 | Scale-Up |
| 3.3 | Manufacturing |
| 3.4 | Storage |
| 3.5 | Homogeneity |
| 3.6 | Miscibility |
| 3.7 | Solubility |
| 3.8 | Formulation |
| 3.9 | Cooking with Cannabis |
| 3.10 | Confections |
| 3.11 | Beverage |
| 3.12 | Sample Analysis |
| 3.13 | Contaminants |
| Chapter 4 | Conclusion |
Quality product development and production begins with understanding ingredients at the molecular level and how those ingredients combine and interact to create the final product. Analytical testing labs and the results they produce are the key to understanding product formulation and manufacturing at that molecular level. If you are reading this guide, you probably appreciate the importance of chemistry throughout these processes and may have also experienced ineffective communication between scientists at testing labs and product developers/manufacturers. To bridge this gap, the authors of this guide have assembled and explained the fundamentals of analytical chemistry into this brief handbook. In publishing this free guide, the authors hope it will become an invaluable educational resource for manufacturers in the cannabis industry looking to effectively leverage lab testing to improve their product development lifecycles and manufacturing quality control systems.
An analyte is a single chemical compound for which an instrument is set to measure. Analytes in cannabis testing are either natural products or impurities. Natural products are the chemical compounds produced by the cannabis plant and include cannabinoids (such as THC & CBD) and terpenes (smell/flavor molecules).
Impurities are not present in the plant naturally, they represent contaminants such as microbials, heavy metals, pesticides and residual solvents. Microbes can grow on cannabis during its growth or storage. Heavy metals may find their way into the plant from fertilizers, soil or water. Cannabis, compared to other plants, has a high propensity to absorb heavy metals from the environment. Trace pesticides, unless applied intentionally, can come from the environment: soil (especially in traditional agricultural areas), water (run-off from nearby fields), or air (overspray from neighbors or due to contamination of the infrastructure).
An instrument must be calibrated in order to detect and measure specific analytes. Calibration is correlating an instrument’s detector output signal to the amount of each analyte that produces the corresponding signal. In order to make this correlation, chemists must use reference standards for each analyte of interest. Reference standard is a pure chemical compound that is either isolated from natural sources or chemically synthesized. By taking these pure reference standards and diluting them to lower concentrations, chemists can construct calibration curves. Calibration curves are lines that mathematically model the correlation between analyte concentration in the sample and the resulting instrument’s detector signal output.
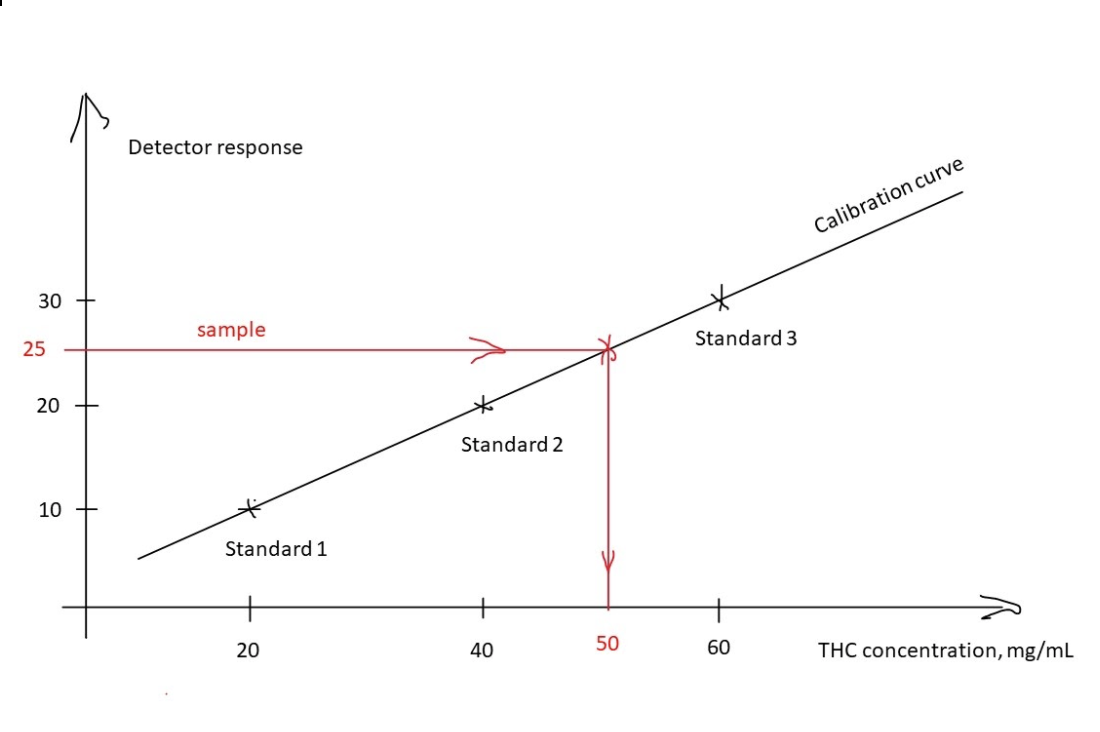
In order to construct a calibration curve, several solutions of reference standard are made at different concentrations. In the example below, solutions at 20 mg/mL, 40 mg/mL, and 60 mg/mL of THC were prepared. Those solutions are called standards and designated 1, 2, 3 in order of increased concentration. When those standards are analyzed by an instrument, the detector response of 10, 20, and 30 respectively was recorded. The three points were plotted on the graph with THC concentration in mg/mL on the horizontal axis (x-axis) and detector response on the vertical axis (y-axis). The line drawn through these three points is the resulting calibration curve. The calibration curve can be used to determine the concentration of the unknown solution based on the detector response. In this example, an unknown sample was run and the detector response of 25 resulted. Using the calibration curve one can determine that this corresponds to 50 mg/mL of THC (red line).
Before samples can be analyzed on an instrument, the analytes of interest must be extracted from the flower, concentrate or infused product matrix into a solvent so that they can be injected into the instrument. This process is called sample preparation. Sample preparation methods are developed for each type of matrix individually, to ensure that all the analytes of interest are removed from the matrix and dissolved in the final solution injected into the instrument. For cannabis flower, the matrix is the raw plant material, for concentrates, the matrix is an oil containing many compounds concentrated from trim/biomass and for infused products (e.g. edibles, tinctures, beverages) the matrix could be a wide variety of things with varying amounts of fats, sugars and other compounds. Each product is a unique matrix because it contains a mixture of many different chemicals depending on the product’s desired characteristics (i.e. thickness, taste, smell, shelf life, color, etc). For this reason, sample preparation is difficult for infused products and ideally each product needs to have a special sample preparation protocol to ensure optimal (ideally 100%) extraction efficiency of the analytes (i.e. THC or CBD) into the solution injected onto the instrument. When potency testing results are lower than expected, this may be due to poor extraction efficiency in the sample preparation protocol, therefore, creating a special extraction protocol for problematic matrices may improve results.
The solution to be injected into the instrument contains many compounds, some are analytes of interest (THC, cannabinoids, terpenes, etc.) and thousands of others that are not analyzed, carry over from the plant. These compounds must be separated in order to detect the analytes of interest without interference by the other chemicals present in the sample. This separation process is called chromatography. Chromatography utilizes differences in analytes’ chemical nature to slow the passage of the analyte through the column of the instrument. Some analytes will travel through the column slower, some faster, and as a result they will exit the instrument at different times. The time it takes the analyte to travel from the injection point to the detector is called the retention time. In order for the analytes to move through the column they have to be carried by a mobile phase which is the solvent which carries the analytes through the instrument. If this mobile phase is gaseous, the analyte is also in the gas phase, and this is termed Gas Chromatography (GC), while in case of Liquid Chromatography (LC) the mobile phase is a liquid in which the analyte is dissolved. Choice of GC vs LC depends on the temperature at which the analyte becomes a gas as well as its thermal stability at this temperature Cannabinoid potency utilizes liquid chromatography (LC) whereas residual solvents and terpene analyses utilize gas chromatography (GC) and pesticide analysis is split between both LC and GC instruments. Since pesticide analysis requires two instruments and is calibrated with the most analytes (66 in California), it also requires much more time and resources to produce the full data set; hence making it the most complicated and costly cannabis analysis test.
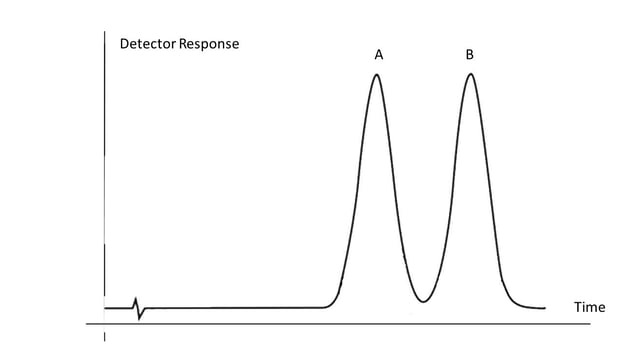
After the analyte leaves the column it enters the detector which produces a signal proportional to the amount of the analyte. Detector is sensitive when it can respond to a small amount of the sample and selective when it can discriminate one analyte from the other. This signal is plotted on the vertical axis (y-axis) against time on the horizontal axis (x-axis) resulting in a chromatogram (see diagram). Tracing the chromatogram above from left to right, for the first two minutes only the baseline noise is observed until analyte A starts being released from the column, peaking around 3 min and then diminished until the detector signal returns to baseline. Next, analyte B is released from the column, peaking around 5 min and is completely washed out of the instrument around 6 min. Analytes A and B are said to be “baseline separated” or completely separated and thus can be quantified using a calibration curve as described above.
The detector must be able to detect the analytes of interest and produce a linear response in order to quantify the analytes of interest. Some detectors are also able to determine purity or even identify analytes. Of the typical detectors encountered in cannabis testing, Flame Ionization Detector (FID) is a nearly universal detector (can detect almost all molecules present) that is highly linear, but cannot determine the identity of the analytes. FID is most popular in cannabis analytical chemistry for terpenes. Diode Array Detectors (DAD) also produces a linear response, but is not a universal detector. DAD detects some molecules but not the others, for example it will detect cannabinoids, but not terpenes. Additionally, it allows an Ultraviolet-Visible (UV-Vis) spectrum for each analyte to be recorded, hence enabling identification of analytes. Mass Spectrometers (MS) are universal detectors that are linear under the right conditions. Mass spectrometers record the molecular mass of each analyte along with its molecular fragmentation pattern which affords high confidence in the identity of each chromatographic peak. Triple Quadrupole MS (TQ or MSMS) is a universal detector that provides unambiguous confirmation of each peak in the chromatogram and also enables a very low level detection as single molecules or single fragments of molecules can be detected. Mass spectrometry is often used for pesticide and heavy metal analysis. MS may also be used for residual solvent and terpene analysis.
The area under the chromatographic peak that is proportional to the amount of the analyte. This “area under the curve” is obtained by “integrating” the peak of interest (a calculus mathematical function) using the software provided with the instrument. The taller and wider the peak is in a chromatogram, the more of that analyte there is present in the sample. The size (area) of each peak is compared to the calibration curve for each analyte created using pure standards/reference materials, thereby allowing chemists to calculate the amount of specific analytes (i.e. THC/CBD or specific pesticides) present in the original sample submitted. These calculations are recorded on a Certificate of Analysis (CoA) which is a formal report indicating the concentration level of each analyte in the originally submitted sample volume or mass. Results for each analyte can be in % of total mass, mg/g, mg/mL, mg/unit, ppm, or ppb depending on the test.
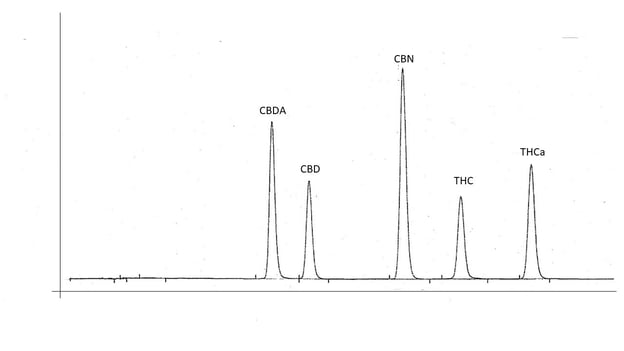
The combination of all parameters from sample preparation through all settings of the instruments and how the output data is analyzed is referred to as an analytical method. The testing of all these parameters in order to optimize performance of the method is called method development. Initial method development can take weeks or months of work. Once a method is established and implemented, it may be modified as needed when improvements to parameters can be identified, or a new sample matrix type does not produce optimal results.
Analytical methods must be validated (method validation) before they can be used in routine testing in order to confirm that they are accurate, reproducible, and reliable. The validation data for an analytical method must fall within certain parameters to be deemed acceptable by both government regulations and internationally accepted lab standards. Both the ISO/IEC 9001:17025 (2017) accreditation process as well as state requirements for lab licensure require that these validation results meet specific criteria. The quality of both validation data as well as daily verification that data is being produced accurately and reliably contributes to data integrity. There are multiple aspects to ascertaining that data integrity is being maintained over the lifespan of an analytical method, including daily instrument calibration and detector signal strength tracking.
Once a method has been validated, it must be compared with comparable validated methods being conducted at other labs to determine if it is producing truly accurate results. This is accomplished through proficiency testing and/or inter-lab testing. ISO/IEC 9001:17025 (2017) and state agencies require that proficiency testing be conducted. Some suppliers of reference materials offer proficiency testing based on the pure reference materials they provide to the industry. A lab that has developed and validated a method for specific analytes can order proficiency testing from these vendors. The vendor then provides samples of the matched matrix spiked with analytes of known concentration. The lab performing the proficiency testing does not know these values and must determine the analyte concentrations using their method. Once the results are returned to the proficiency test provider, they will issue a pass or fail result based on the known analyte levels in the test material. If a proficiency test from a commercial vendor for an analyte does not exist, the method needs to be confirmed as accurate by performing inter-lab testing wherein another analytical chemistry lab using their method concurs results with the new lab/method. Labs must pass all proficiency tests (if available) or demonstrate inter-lab concurrence for all analytes in their scope of accreditation to comply with ISO/IEC 9001:17025 (2017) requirements on an annual basis.
Once an analytical method has been validated and passes proficiency and/or inter-lab testing, it can be conducted on a daily basis. Before every batch is run, a lab must perform daily system suitability checks to ensure the system is operating within specific parameters. During the run, at prescribed intervals, a Continuing Calibration Verification (CCV) needs to be conducted to ensure a standard and the calibration curve are consistent and that the instrument remains in calibration throughout the day. CCVs are simply samples of reference materials of known concentration. These CCV values must match to the appropriate point on the calibration curve within a limited range of error in order to be considered valid. If a CCV fails (i.e. fall outside accepted limits), no further samples can be analyzed until the instrument is brought back into calibration. CCVs can be tracked over time to assure consistency of the method and performance of the instrument over a period of time. By plotting these daily CCV values on a control chart, a lab can determine when an instrument falls out of specification and needs to be cleaned and/or serviced. These activities may force a lab to stop analyzing samples for one or several days until the instrument can be brought back into calibration and operation.
Upon completion of analysis, chemists review all the data generated by the instruments and send them to an analyst who verifies the data interpretation and releases them into the Laboratory Information Management System (LIMS). This process is called data Quality Assurance (QA) review. Redundant data review ensures that data processing protocols are followed consistently among all staff and to double check that no human errors were made during the data processing and final sample concentration calculations that appear on the COAs. Good data reporting practices require at least one secondary review of results before releasing a COA, and it is even better practice to have a third (tertiary) reviewer approve the data before a COA is issued. The final reviewer of the data is usually the signature of the person on the COA.
Concentration refers to the amount of mass of one analyte distributed throughout the volume of a sample. By analogy, population ‘concentration’ is measured as the number of people living within a square mile. For example, Malibu, CA has a density of 645 people per square mile whereas Manhattan, NYC has a density of 70,826 people per square mile. Manhattan is therefore about 110 times more densely ‘concentrated’ in population than Malibu. Concentration in chemistry is defined as an amount (mass) of an analyte distributed within a given volume or mass of the sample. Typical concentrations are mg/g for potency, ug/g (or ppm) for pesticides, and ng/g (or ppb) for heavy metals, where ug is microgram 1/1,000,000 (1 part per million, ppm) of a gram and ng is nanogram 1/1,000,000,000 (1 part per billion, ppb) of a gram. If the solution is a liquid, mg/mL or mg/L are typically used, where mL is 1/1,000 of a liter.
From the instrument output chromatogram, the area under the curve is calculated for the analyte for interest, and that area is then applied to the calibration curve to calculate the concentration of the analyte in the original sample provided. Typical cannabis sample will have, in order of decreasing concentrations, cannabinoids (mg/g), terpenes (mg/g), residual solvents (ug/g = ppm), pesticides (ug/g = ppm), and heavy metals (ng/g = ppb). The thousands of other naturally occurring molecules as well as other environmental contaminants that compose the rest of the sample are not measured.
When reporting cannabinoid and terpene potency percentages, it is important to use the dry mass of the sample for flower analysis as water content varies between products. The lab doing a potency analysis will calculate the percentage of water in a sample before calculating potency percentage to ensure that the water mass is not included in the potency calculation.
In a sublingual (under the tongue) or buccal (between cheek and gums) administration, as with lozenges, tinctures, or hard candy, THC gets absorbed into the bloodstream directly from the mouth, again bypassing the liver resulting in the effect that is 3 times faster than when THC is ingested. The downside is that the product has to spend enough time in the mouth to be completely dissolved. It’s fast with a tincture but can take quite a bit longer with a hard candy.
In case of ingestion, the product has a long way to travel from the mouth to the stomach and then the intestines where THC (tetrahydrocannabinol) is finally absorbed and then transported into the liver. The liver converts (metabolizes) THC into 11-hydroxy-THC which enters the bloodstream. The process takes 30-40 minutes and only a fraction of TCH originally present in the product is absorbed. On the other hand, 11-hydroxy-THC is about 5 times more potent than parent THC so the effect is stronger and somewhat different than with other routes.
Traditional topical application (directly on the skin) does not allow THC to reach the bloodstream, but a transdermal application, literally meaning “through the skin”, does. A transdermal patch contains special chemicals (such as DMSO) that allow THC to travel through the skin and into the bloodstream.
Cannabinoids, along with terpenes are biosynthesized in specialized resinous glands, called trichomes (capitate stalked and capitate sessile) which concentrate in the flower and sugar leafs. Cannabinoid production starts with CBG (cannabigerol) which is converted inside the plant into CBDa (cannabidiolic acid) and then THCa (tetrahydrocannabinolic acid). Over time THCa decomposes to CBN (cannabinol). CBG itself is made out of two components: a terpene (geranyl phosphate) and a phenol (olivetol), which are also synthesized in the plant. Cannabinoids and terpenes accumulate in the top part of the gland (called the cap), while the stalk contains neither. Recent research identifies the top part of the cap as the location where cannabinoids are stored, while terpenes are found in the bottom part of the cap. Plant uses terpenes to stave off predators as many other plants do, while the purpose of the cannabinoids is yet unknown. Cannabinoids are also found in other, non-cannabis, plants: clove, black pepper, Echinacea, broccoli, ginseng, and carrots (!)
Humans discovered the beneficial properties of the cannabis plant over 2000 years ago. Originally, it was used only in a form of hashish (aged resin that was collected directly from the plant) but in more recent times smoking dried flowers became the primary mode of THC consumption. For decades cannabis was formulated into a variety of foods such cookies and brownies, as new processing technologies became available, additional product categories emerged: concentrate for dabbing, oil for vaping, and a wide variety of edibles, infused beverages, and personal care products.
After cannabis is harvested it’s either hanged to dry or frozen. Dried (cured) cannabis is processed to separate the flowers from the rest of the biomass. Flowers are trimmed to remove small leaves (called trim) and packaged for sale. Leftover biomass is further processed to recover remaining THC. Frozen flowers, called fresh frozen, which, unlike cured flowers, preserve all of their terpenes, are further processed to make a full spectrum product. Flowers can be ground and packaged into capsules for internal consumption.
The simplest and most direct way to obtain cannabinoids from the cannabis plant is by pressing the cannabis oil out of the buds using a heated press. The plant material is put into a special bag between two sheets of parchment paper and positioned between the platens of the press. High pressure (5-25 tons, depending on the amount) and heat (200F) are applied to squeeze the flower rosin which is typically darker in color. Hash rosin, which is lighter in color, can be made from kief or bubble hash at lower temperature (120F) and pressure. Typical mass recovery of rosin is about 25% of the original flower by weight (from 100 g of flower about 25 g of rosin is expected), or about 90% of the original fresh-frozen hash weight (from 100g of fresh frozen hash 90 g of rosin is expected).
Trichomes, which are strongly attached to the living plant, fall off the harvested plant during handling, especially after it has been cured. Using agitation, trichomes can be removed from the plant (buds and leaves) and purified using a screen to remove leaf fragments yielding kief. Kief color varies from light gold for very pure kief to greenish for kief contaminated with plant material. Kief is made into hash by pressing, during which cell walls are broken and cannabinoids and terpenes are released. Dry ice (frozen CO2) can be used to cool the plant material to facilitate the breaking off of the trichomes which become more brittle when cold, screening with a filtration bag yields dry ice hash. About 36% (by weight) or trim is recovered as kief this way. Trichomes do not dissolve in water, so ice water has been used to make solventless hash. Ice water simultaneously makes trichomes more brittle and creates shear force aiding in their separation from the plant material. A filter bag system (aka bubble bag), which is an assembly of screens of increasing mesh size, is used to separate trichomes from ice water and plant material resulting in water hash (aka bubble hash). Excess water is removed using a freeze drier or by carefully blotting and air drying the wet hash. Water hash production method can be scaled to up to 5 lbs of starting plant material using a machine. After hash is thoroughly dried it’s pressed into a pliable mass using pressure and low heat. Hash is dabbed, made into tinctures, infused into edible oils for cooking, or pressed into rosin. On average 10% of weight is recovered as water hash which contains about 70% cannabinoids. Starting with 100 g of plant material one would obtain 10 g of water hash containing 7 g of cannabinoids. If the starting plant material contained 25% (25 g) of cannabinoids, 7 g (28%) of cannabinoids will be recovered, thus an average cannabinoid recovery of water hash extraction is around 28%.
Filtration is a physical separation process where the mixture of liquid and solid is passed through the filter. The filter can be a fine metal mesh, sintered glass, an inert powder, or a filter paper. The liquid passes through the filter while the solid particles stay behind as they are too big to pass through the channels in the filter. The liquid that passes through the filter, called filtrate, is thereby free of solids.
To recover remaining cannabinoids and terpenes from the leftover biomass, organic solvents are used in a process called extraction. In the extraction process a solvent (ethanol, butane, or carbon dioxide) is used to dissolve the trichomes containing cannabinoids and terpenes and thus separate them from the plant material. The solvent is then evaporated (ethanol) or purged (butane and carbon dioxide) and what remains is the mixture of cannabinoids, terpenes, and small amounts of undesirable plant chemicals (plant lipids and waxes) and contaminants (pesticides, heavy metals, etc). Since contaminants are concentrated from the starting biomass and extraction solvents, ingredients of highest possible purity should be used. Extraction process can be fine-tuned to optimize a number of parameters: safety, throughput, recovery, purity, etc.
Butane is the most popular extraction solvent, it produces the highest purity extract with very high recovery, but offers a lower throughput compared to ethanol and CO2 and is much more dangerous due to high flammability. Either cured or fresh frozen biomass can be used as a starting material. Butane boils at 30F and it’s pressurized to keep liquid inside the source tank, when it’s allowed to expand into the extractor it cools so the extraction occurs at a very cold temperature. When making live resin, butane can be cooled further before extraction as the colder it is the more selective is the extraction towards cannabinoids and terpenes and the less residual water interferes. The resulting extract can be theoretically purged in an open system allowing butane into the surroundings but it’s very dangerous as butane is extremely flammable and can easily ignite. Instead, butane is purged in a closed system where it’s recovered into another tank for reuse. It’s recommended that the recycled butane is tested periodically to reduce the risk of contamination of subsequent batches should the previous biomass prove to be contaminated. What remains after purging is called BHO (butane hash oil) which contains on average about 70-80% THCa. When cured biomass is extracted, the product is called cured resin, while fresh frozen buds yield live resin (aka full-spectrum extract). Full-spectrum refers to complete recovery of all terpenes from the original plant. Depending on how butane is purged, different physical forms of BHO are obtained: wax is of taffy consistency, shadder is glassy and translucent, budder shows nucleation in a glassy matrix, butter or crumble is microcrystalline, sugar is of visible crystals, and diamonds are big crystals of THCa. Fresh frozen material extracted at very low temperature yields sauce, aka HCFSE (High Cannabinoid Full Spectrum Extract). The process of growing diamonds from the sauce is called diamond mining (chemists call it recrystallization, a deliberate formation of large pure crystals): THCa is allowed to crystallize slowly onto a few seed crystals allowing a few crystals to reach a large size. In contrast, if crystallization is allowed to occur quickly, too many seed crystals form at the same time and don’t have a chance to grow resulting in a microcrystalline material. After diamond mining, about 30-50% of cannabinoids and most of terpenes remain in what chemists call mother liquor referred to as HTFSE (High Terpene Full Spectrum Extract).
Recrystallization is a purification process, whereby the desired solute is allowed to crystallize slowly, typically induced by cooling and/or change in the solvent composition, typically by allowing a more solubilizing solvent to evaporate over time. Slow crystallization yields bigger and purer crystals, while the remaining liquid from which the crystals grew is called mother liquor.
Carbon dioxide (CO2) extraction is the safest of the three options as it is not flammable, but is used under high pressure and if large amounts are released into a poorly ventilated room at once, no oxygen might be left to breathe. CO2 extractors offer much higher throughput compared to butane but are not suitable for small loads and the extraction process is significantly slower. While the recovery of cannabinoids is comparable, the process needs to be well tuned to allow for recovery of terpenes. Material used for CO2 extraction, typically leaf and trim, must be relatively dry (less than 10% water) to avoid deleterious side reactions. Resulting concentrate is about 50%-70% THCa, the purity can be improved to 80%-90% by dewaxing via winterization, but terpenes are lost in that process. Typical CO2 shatter contains about 70%-80% THCa. CO2 oil is used for filling vape cartridges.
Winterization is a process whereby the extract is dissolved in hot ethanol and upon cooling, the undesirable fats and lipids precipitate out. The solution is filtered and ethanol evaporated to yield a cleaner cannabis oil.
Precipitation is a physical separation process, typically induced by cooling, whereby less soluble, and often undesirable, analyte crystallizes out of the solution. As Brits say, if you are not part of the solution you are part of the precipitate.
Cold ethanol extraction is safer than butane, but ethanol is still very flammable. Cold ethanol extraction can be done in standard chemical reactors and so it can be scaled to massive scale, the bottleneck becomes the amount of ethanol needed and the availability of large-scale ethanol recovery equipment. Ethanol extract often has to be processed further through winterization (removal of plant lipids) and distillation (purification by boiling and re-condensing).
Distillation is a physical separation process whereby a mixture is heated to volatilize the component of interest which is then re-condensed and collected separately from all other components. Short path distillation refers to the short distance the vapor travels before condensing, the apparatus is so designed to minimize excessive heating needed to help propel the vapor along a longer path as well the loss of desired material on internal surfaces. Fractional distillation refers to collecting distillate into separate fractions, from lightest to heaviest, with desired THCa fraction being in the middle. In wiped film distillation the oil is run along the walls of a heated tube under low pressure. The heat causes THC to evaporate and separate from residue which runs down the tube and is flushed out. The THC distillate is condensed and collected in a separate container.
Crude oil produced by one of the extraction techniques above that is otherwise not suitable for direct consumption can be processed into a distillate, which is a decarboxylated THC oil of about 90% purity. Distillate is often reformulated with recovered cannabis terpenes or with a blend of botanically-derived terpenes for use in vape cartridges. They are also used directly for dabbing or infusing edibles.
Decarboxylation is a chemical reaction that converts natural, non-psychoactive, THCa into psychoactive THC. The term “decarboxylation” stands for a loss of carboxylic acid group in a form of carbon dioxide which can be seen bubbling out during the process. The “a” in THCa stands for the presence of this carboxylic acid group, not present in the THC. Decarboxylation occurs above 222 degrees Fahrenheit.
Tincture is a solution of decarboxylated cannabis oil in aqueous alcohol (25% alcohol in water), MCT (medium-chain triglyceride) oil, or glycerin. Tinctures can be made directly from the buds by extracting them with alcohol and then diluting with water, or by dissolving decarboxylated cannabis oil in the solvent of choice. Tinctures are taken directly by mouth and are absorbed quickly into the bloodstream. Tinctures can also be used as a source of THC in cooking and making infusion products.
Transdermal patches are used extensively in the pharmaceutical industry to slowly deliver a drug through the skin. An existing technology was adapted to making patches containing THC or CBD. Unlike topicals patches deliver THC through the skin into the bloodstream for full effect. The rate of delivery can be designed to achieve a desirable result.
Infused products are produced by adding THC oil as one of the ingredients during manufacturing a product from scratch or infusing it to a pre-made product. Infused products can be broken down into several categories: topicals and personal care products, edibles (baked goods, confections, etc), and beverages. Infused products were made with cannabis for a long time (brownies or cookies for example) and are the fastest growing cannabis product category today.
Topicals personal care products such as creams, lotions, salves, balms, etc. are applied externally, releasing THC that is coming into contact only with the skin, not entering the bloodstream, leading to a mild effect and the absence of euphoria.
Edibles, the largest category of infused products, is composed of THC-infused processed foods such as baked goods, salad dressings, chocolate, mints, gummies, hard candy, bacon, etc. When baking with cannabis temperatures are high enough to cause in-situ decarboxylation activating the THC. Cannabis source is typically an infused butter or edible oil that mixes well with the rest of the ingredients and doesn’t add an undesirable flavor to the product. Making confections (chocolate, gummies, hard candy, mint) is an art in-and-of-itself, and adding cannabis makes the manufacturing so much more challenging. Proper dosing; cannabis distribution throughout the product (homogeneity); product stability such as oiling out, moisture absorption, solids migration (in chocolate), etc are just some of the examples.
Beverages -- THC is lipophilic (dissolves in other oils) and does not dissolve in water. Since all beverages are composed primarily of water, formulating THC into a beverage is very challenging. If THC is added directly to a beverage, it will not dissolve, but instead oil out, creating a thin layer of oil near the cap. One way to dose THC into a beverage is by creating an emulsion, a metastable suspension of oil in water with the help of an emulsifier. In the emulsion emulsifier molecules surround a lipophilic molecule of THC such that their hydrophilic (water-loving) groups are exposed to the water allowing it to stabilize. Emulsion droplets are too small for the eye to see clearly so they appear as haziness making the solution non-translucent. The size of the emulsion droplets is very small and so the beverage is often referred to as a nanoemulsion. Exact identity of emulsifiers the given company uses are often proprietary, but there are plenty to choose from in the food industry. One example is lecithin which is fairly common.
References: Ed Rosenthal’s Marijuana Grower’s Handbook 2010 Quick American Publishing;
Ed Rosenthal with Greg Zeman Beyond Buds. Next Generations. Cannabis Concentrates, Extracts, and Marijuana Infusions.
Before a product reaches the market, it must go through several stages of development in order to produce a consistent and quality product. The first step in the product development lifecycle is proof of concept. In this stage, the product is formulated based on the company’s expertise and market opportunity. Several factors are important to consider here: type of product (i.e. gummy vs chocolate), dosage (5 mg vs 10 mg per serving), cannabinoid composition (THC, CBD, or both), unit size, packaging, etc. Next, representative samples are made on a small scale in an iterative way using different ingredients, flavorings, colorings, sources of THC etc. At this stage of product development small kitchens are often used, along with standard cooking equipment and supermarket ingredients. The best recipe is taken forward to scale up at bench scale. It’s important to partner up with a lab at this stage to understand how the potency of the raw material is converted to the potency of the final product and what is the rough distribution of potency in the final form.
Once the recipe has been finalized on a small scale, the manufacturing process must be transferred into a commercial kitchen facility and scaled up to fulfill the market niche. At this point professional equipment is used, commercial ingredients of consistent quality are sourced and tested for contaminants, and process parameters, such as source and amount of THC, mixing time and temperature, are optimized. In-process controls (IPCs) are developed to assess the progress of the batch. One such control is the amount and distribution of the THC in the sample. When the distribution is found to be uneven or the amount of THC is not as expected, various correction strategies can be devised (additional mixing time, lower or higher temperature, different carrier for THC, etc. It is important to have a lab partner at this stage that has fine-tuned its method for the exact sample, can recommend meaningful IPCs, and help interpret the data in the context of the scale-up challenges still to be addressed.
When scale-up is done right, production at scale is highly predictable and risks of failure are minimal. Quality assurance plans must be put in place during this stage, including standard operating procedures (SOPs), raw material qualification, batch logs, IPCs, and cleaning logs. If different equipment is used at this scale, small adjustments might have to be made. Temperature, mixing time and speed, and dosing process are typical parameters to consider. Quality control acceptance criteria for the final product have to be developed, and may include color, appearance, texture, smell, taste, unit weight distribution, potency, etc. Ideally the final bulk product is tested before packaging to avoid surprises. The lab partner must be able develop an adequate sampling protocol that will be highly predictive of the final compliance testing.
Shelf stability is the time the product can be on shelf without degradation. An accelerated stability study, done at higher temperature and humidity than is typical for warehouse conditions can simulate and predict stability over longer periods of time under regular (actual) storage conditions. To obtain more accurate shelf-life stability lifetimes, the product may be stored under typical warehouse conditions and is monitored via regular lab testing over a period of time until degradation is observed. Degradation implies that the product no longer meets QC requirements.
Homogeneity refers to the equal distribution of an analyte throughout the entirety of a sample. When a chemical is found to be in the same concentration regardless of which part of the sample is analyzed , that sample is said to be homogeneous. A plain sugar cookie, for example, would be considered homogeneous because regardless of which cookie from a batch or which part of an individual cookie one was to sample, analysis would reveal the same sugar content (concentration). By contrast, raisin cookies would not be homogeneous (hence, heterogeneous) because each cookie in a batch or each bite of an individual cookie would contain a different number of raisins. In the cannabis world, homogeneity is important because the dose that a consumer will receive upon consuming a single serving of a product must correspond to the label claim. If the product is heterogeneous with respect to THC, some of the product will be below the label claim and some above; hence, consumers will experience different effects based upon which part of the product they consume. For this reason, products must be made homogenous so that consumers experience consistent effects when they consume a defined serving size of any infused product.
Plant material is naturally heterogeneous but it can be homogenized by grinding and mixing into a fine powder of uniform consistency. This is, in fact, the first step of sample preparation in the lab when a sample is submitted for testing. Upon successful homogenization, the THC content (as well as other analytes) is evenly distributed throughout the entire sample and is consistent regardless of how the sample is separated and distributed from that point forward.
Concentrates appear homogeneous to the naked eye, but the appearance may be deceiving. Often, the concentrate is collected continuously throughout the separation process, with the newer fraction(s) deposited on top of the older one. Because concentrates are typically very viscous, those layers would not mix without agitation, so the sample as a whole might be heterogeneous. For this reason, when a batch is sampled from the top, the analysis might not be representative of the entire batch (i.e. bottom layers). For this reason, it’s highly recommended that the entire batch be mixed before testing and packaging for sale.
Infused products are complex mixtures of many ingredients with widely varying affinity for THC, CBD and other cannabinoids. Dosing them in the right amount and at the right time, mixing adequately to ensure equal distribution of THC throughout the batch, and proper parameters to avoid THC oiling out during the process are all important factors to consider. From a lab testing perspective, sample homogenization, extraction and preparation protocols must be developed for each type of product matrix in order for valid test results to be generated. Oftentimes standard methods that work for some product matrices, simply don’t work well for others due to the wide range of different ingredients between products.
When combining ingredients to create infused products, it is essential to ensure that ingredients mix well together and therefore are miscible. Miscibility refers to the ability of liquid chemicals to mix completely together and not separate once mixing stops, thereby ensuring the mixture can be made and will remain homogeneous throughout the process. There are two types of liquids: oil-based and water-based. Generally, oil-based liquids are miscible with each other and likewise water-based liquids are miscible with each other, but oil- and water-based liquids are NOT miscible with one another. For this reason, cannabinoid concentrates should only be mixed with other oils such as cooking oils (i.e. olive, canola, vegetable, coconut, etc), butter, etc. Cannabinoid concentrates should never be mixed directly into water as they will not combine into a homogenous mixture, rather the concentrate oil will stick to the sides of the container, create oil droplets suspended in the water, and/or rise to the top creating a separate layer. However, water and oils can be mixed together with dry solid ingredients to make batters or doughs. It is critical that a producer take exceptional care to make sure that any and all mixing throughout the processing, especially when mixing wet and dry ingredients together, is done thoroughly to guarantee homogeneity. Visual cues can clearly indicate when a mixture is not homogeneous, but does not guarantee that it is homogenous. There is typically no harm to mixing too much, but there is risk of producing a heterogeneous product if mixed insufficiently. In other words, more mixing is almost always better than less.
Solubility refers to the ability of a solid to mix into a liquid to create a one-phase solution, which is homogeneous, without separate layers or precipitate. Before mixing solid concentrates into cooking oils, it is advisable to break them into as small pieces as possible before mixing as this will reduce the amount of mixing necessary to completely dissolve the concentrate in the oil. Failure to do so may result in some portions of the batch having higher THC/CBD concentrations, hence setting up the manufacturer for a label claim testing failure when going to market. Solid concentrates should always be dissolved into cooking oils before mixing with dry ingredients to ensure homogeneity is achieved in the final product. It’s difficult to obtain a homogeneous mixture of solids unless they are both a fine powder. Because cannabis concentrates are often ‘sticky’ they cannot be mixed well with other solids.
In order to obtain a properly formulated product that meets label claims, the right amount of THC must be added to the product formula and all ingredients must be thoroughly mixed to ensure homogeneity.
In order to formulate the desired amount of THC into an infused product one needs to know the concentration of THC in the concentrate being used, the mass of the concentrate used, the total mass of the final product, and the mass of individual doses. Thus if the concentrate has 50% THC by weight and 10 g of concentrate is used, 5 g of THC is contained in it and is infused into the product. If the total mass of the final product is 1000g then the 5 g of THC is distributed in the 1000g of the product. If there are 1000 doses (1 g each), there is 0.005 g (5 mg) of THC in each dose.
In order for this approach to work, THC has to be evenly distributed throughout the batch. If it’s not, some doses will end up with more THC and some with less and as a result the whole batch will fail compliance testing for potency label claims. Even distribution of THC is achieved through efficient mixing of all the ingredients as well as understanding the stability of metastable states. Metastable state is a state which is stable only under certain conditions and only for a limited period of time. An example is the hard candy mixture which, when overcooked, separates into the candy layer and the THC oil layer. In this case, the candy layer gets thicker with heating, as sugars polymerize, and the THC oil layer becomes less and less soluble until it separates.
Temperature and time that are important in cooking are also very important in considering the integrity of the cannabis components in the resulting edible. There are two processes to consider, evaporation of ingredients (terpenes and THC) and chemical transformations (decarboxylation, THC to CBN conversion).
THC has a boiling point of around 425F, but in the open system it begins to evaporate at above 320F. It is not recommended to exceed 350F when making edibles. Please note, that when the pressure in the system is reduced (vacuum is created), as in a short path distillation when the vacuum pump is used, the boiling point of the components is reduced correspondingly.
Every chemical reaction has an activation energy, meaning there is a minimum temperature required for the reaction to start. The rate of the reaction depends on temperature, the higher the temperature the faster the reaction. A good rule of thumb is for every 20F above the activation energy the reaction rate doubles, so if decarboxylation takes 1 h at 220F, it will take 30 min at 240F and 15 min at 260F. Below the activation energy (220F) the rate of decarboxylation will be much slower if noticeable at all. Increasing the temperature also increases the rate of terpene evaporation, which must be taken into consideration for edibles wishing to retain the terpene smell/flavor profile.
During the edible manufacturing one needs to decide if the cooking temperature is sufficient to cause spontaneous decarboxylation or a separate decarboxylation step is needed to make sure all THCa from the plant is converted to THC. Typically, in baking, temperatures above 235F are reached and thus decarboxylation occurs spontaneously. When one is baking no prior decarboxylation step is required.
Conversion of THC to CBN, (a non-euphoric, sedative compound) takes weeks at room temperature, but hours at 300F. For this reason, it is recommended not to cook an edible above 300F to avoid converting a psychoactive THC product into a sedative CBN product.
THC is typically incorporated into a recipe via infused butter or oil. Alternatively, hash, rosin, tincture or distillate can be used. Cooking with raw cannabis directly is less common as it significantly impacts the taste and texture of the final product which most consumers find unappealing.
Olive oil, canola oil, coconut oil, butter, or hemp oil infused with THC are all used in cooking edibles. Infusion is accomplished by cooking the oil with decarboxylated trim or buds at low heat (225-250F) for several hours to days followed by straining to remove the plant material. Alternatively, the oil can be fused with rosin using medium heat. The later process is much more efficient as the THC recovery from the rosin is much higher than from trim or buds. Is it ok from wax? Double-boiler into coconut oil
Candy is currently the most popular infused product on the market. There are many types of candies currently available: gummies, hard candies, and mints are likely most popular. In all of these formulations, sugar is one of the main ingredients. It’s mixed with other ingredients and heated to obtain the right consistency. Achieving homogeneity, avoiding separation, dosing the right amount of THC, achieving consistent weight of each serving, and maintaining stability of the final product all the way through packaging are the major challenges.
Gummies are made from fruit juice, sugar, and gelatin. Ingredients are combined at low heat, transferred into mold and cooled. The gelatin polymerizes as it dries, thereby giving gummies their chewy texture. It’s recommended to use THC concentrate when formulating gummies as the temperature used in the manufacturing process may be insufficient to cause decarboxylation to convert THCa to THC. To get the THC/CBD oil to mix completely with the water/juice emulsifier have to be used.
Hard candies are made from sugar, corn syrup, water, and flavorings. THC can be added as an infusion in the butter or as a tincture. The mixture is slowly heated while stirring until it reaches 300F. In the process, water boils out and the mixture turns thick and brown as sugars polymerize. Once the mixture reaches 300F, it is transferred into silicone heat-resistant molds and allowed to cool. Once at room temperature, candies are separated from the mold and packaged. Since the cooking temperature exceeds decarboxylation temperature (220F), a THCa infusion or tincture can be used as it will decarboxylate to THC spontaneously during the cooking process. When flavorings and other natural ingredients are used, it’s important to ensure that they don’t contribute forbidden contaminants such as pesticides or heavy metals.
Mints are made from gumpaste mix, sugar, and flavorings. Gumpaste is made from sugar, gelatin, water, white vegetable fat, egg white, and Gum Tragacanth or CMC. Gum Tragacanth is a mixture of water-soluble polysaccharides from a plant.
Chocolate can be made from scratch or by melting bulk chocolate and mixing in THC. Chocolate is made from chocolate liquor (mixture of cocoa butter and cocoa solids made from cocoa beans), cocoa butter, sugar, lecithin, and vanilla.
If THC or CBD and water are mixed together an unstable emulsion forms which will separate into layers upon standing. However, when a special stabilizing agent (an emulsifier) is added, the suspended droplets of THC/CBD will float indefinitely in the water, thus preventing separation of the oil (THC/CBD) and water layers. Milk is an example of a naturally stable emulsion, composed of small fat globules suspended in water. An unstable emulsion separates into two layers, like when trying to mix olive oil and vinegar. In a salad dressing, oil and vinegar emulsions can be stabilized, typically with the help of lecithin emulsifiers.
Several emulsifiers for stabilizing THC-containing drinks have been developed. As a result, a variety of infused drinks are available on the market: water, soda, “beer”, “wine”, etc. Since alcohol cannot be legally combined with THC in one product, the “beer” is actually an infused hop water, and “wine” is infused non-alcoholic wine. Hop water is obtained by boiling spent hops from the beer manufacturing process in water, and non-alcoholic wine is produced by boiling ethanol out of regular wine. Nanoemulsion refers to an emulsion where the individual droplets of THC oil are of nanometer width (one billionth of a meter), hence the drops cannot be seen with the naked eye.
Analysis of an edible sample begins with homogenization, typically in a blender. The homogenized sample is then extracted using an organic solvent. During the extraction, all compounds of interest, including cannabinoids migrate into the organic solvent while the insoluble matrix compounds remain behind in the solid. The solution is then filtered and injected into an HPLC where the separation between individual components of the sample is achieved. The detector measures the amount of each component (analyte) by comparing the response to the calibration curve. In this manner, the exact amount of THC in the original homogenized sample can be determined.
The product matrix has significant effects on the accuracy of lab testing. Some matrices (such as gummies) do not homogenize easily in a blender and hence alternative methods may be required. More intense cooling, or, alternatively heating can be employed to improve the homogenization process. Different mixing equipment can also be employed to improve the process. It’s absolutely essential to achieve complete homogeneity in a sample in order to accurately determine the average amount of THC in any infused product by serving size.
Some matrices are less cooperative in releasing THC under standard extraction conditions. Since it’s not obvious when an extraction is complete, additional experiments, called matrix recovery studies must be performed. To remedy incomplete extraction, conditions must be optimized to obtain maximum extraction efficiency that will produce analytical results indicative of the true THC content of the sample. In some cases, it’s as simple as using more solvent relative to the amount of sample, whereas in other cases a different solvent composition may work better, and in other cases additional clean-up steps may be required.
Residual amounts of matrix are always present in the extracted sample, which may interfere with downstream separation and measurement within the analytical instrumentation. In this case, additional sample purification steps are necessary prior to HPLC analysis, or the HPLC method may need to be fine-tuned to ensure separation of these interfering analytes.
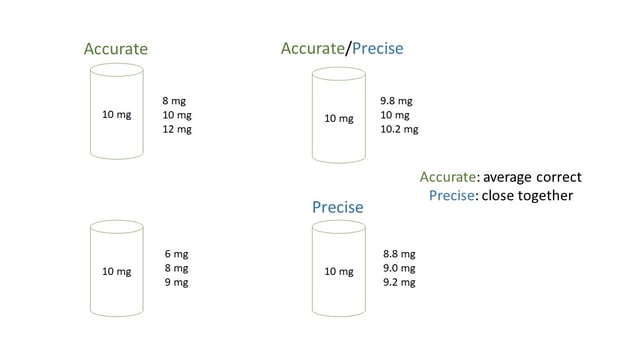
Accuracy and precision of cannabinoid measurement is affected by a number of factors. Accuracy is a measure of closeness to the true value. Think of hitting a target, the farther a projectile is from hitting the bullseye, the lower the accuracy of that shot. Precision is a measure of closeness of repeat measurements to the same value. If multiple projectiles strike the target near one another, the shots are precise. Measurements can be precise but not accurate or accurate and not precise. Different factors contribute to each. In the diagram below three measurements are taken, in each case the sample has a label potency of 10 mg. In the first examples (top left), measurements are accurate (the average is spot on) but not precise (difference between measurements is too high), on the bottom right three measurements are close together (precise) but the average is off (not accurate). The measurement on the top right is both accurate and precise and the one on the bottom left is neither accurate nor precise.
In the testing lab, if extraction efficiency is 100% and an analytical instrument method is highly reproducible, results will be both accurate and precise. Conversely, if the analytical method is not reproducible due to an aging system lacking proper maintenance, measurements may sometimes be accurate but will not be precise.
In order for a test method to provide meaningful information from sample analysis, the accuracy and precision of the method must be fully understood. This is achieved through method validation, proficiency testing, and inter-lab testing (see Chapter 2). Method accuracy is ensured by measuring extraction efficiency, measuring surrogates of known concentration, and participating in inter-lab testing with other legitimate labs. Even when all those precautions are taken, a systematic error (one that occurs consistently) may negatively affect the result. To mitigate such a risk, a deep understanding of chemistry must be applied in developing custom methods for each type of infused product. Method precision is measured using replicate injections from the same sample. Precision is expressed as percent relative standard deviation (%RSD) which indicates how big of a spread those replicate measurements span. A method with a small %RSD, is therefore highly precise.
In one scenario, to determine homogeneity of a bulk batch three samples were taken: two from the top and one from the middle. The results were 10 mg/g, 9.5 mg/g, and 10.2 mg/g of THC. The %RSD of these three measurements is 3.6% which is less than the method precision of 4.3% RSD as reported by the lab. Therefore, with this sampling method and analytical method, it can be concluded that the bulk sample is homogeneous.
In another scenario, four samples were taken: two from the top, one from the middle, and one from the bottom. The results were 10 mg/g, 9.5 mg/g, 10.2 mg/g, and 12mg/g of THC. The four measurements have 10.5% RSD which is higher than the methods. Therefore it can be concluded that the sample is not homogeneous (beyond the uncertainty of the method).
Contaminants are defined as any unwanted, non-naturally occurring chemicals or microbials in a sample. Most states requiring cannabis testing agree that contaminants need to be checked for foreign materials, heavy metals, pesticides, residual solvents (for concentrates) and microbiological impurities (bacteria and mold).
Foreign material includes any filth visible to the naked eye or with minimal magnification (i.e. macroscopic, not microscopic). Common types of filth include hair, rodent excrement, dead insects, dirt and mold. In the state of California, a sample will fail foreign material inspection if it visually contains more than 25% of its surface area covered in filth. Historically as of April 2020, less than 0.5% of samples submitted for compliance testing in California failed foreign material inspection.
Heavy metals are some of the largest elements, generally located in the middle section of the periodic table. Heavy metals are generally known to be toxic to humans, particularly from a neurological perspective. The state of California requires testing for Lead, Arsenic, Cadmium and Mercury at very low levels as low as 0.1 ppm (micrograms of metal per gram of sample). The cannabis plant is excellent at absorbing metals from its soil or growth medium, and in fact, the cannabis plant has been used to clean-up polluted land due to its propensity to accumulate heavy metals. About 10% of cannabis compliance testing failures in California occured due to heavy metal contamination as of April 2020.
Residual solvents are chemicals used in the processing or extraction of cannabinoids from biomass material into oil concentrates. These chemicals must be removed (usually via evaporation) before the oil or product is safe for consumption. There are 20 solvents that products must be tested for in California, and much like pesticides, they are divided into the more harmful category 1 solvents (6 analytes) and less harmful category 2 solvents (14 analytes). Residual solvents are responsible for 5-6% of the state of California’s cannabis compliance testing failures as of April 2020.
Pesticides are the most common contaminant responsible for compliance testing failures in California, representing about 30% of all failures as of April 2020. The state of California mandates that 66 different pesticides be assessed in cannabis products, separated into category 1 and category 2 pesticides. There are 21 category 1 pesticides (the more toxic ones) and 45 category 2 pesticides. Each pesticide has a specific allowable limit with the lowest being 100 ppb (ppb = one billionth of a gram of pesticide per gram of sample) for all category 1 pesticides and some category 2. Some category 2 pesticides levels can be 100-400 times higher than others and still acceptably pass state compliance testing.
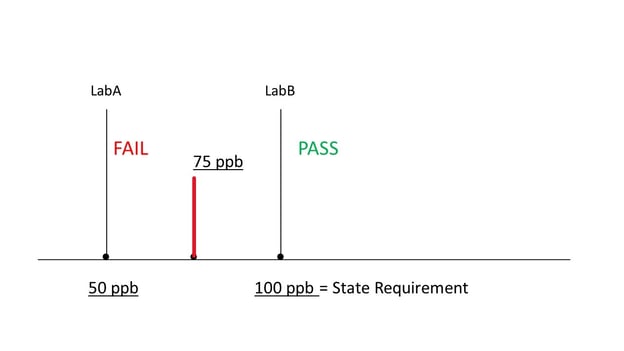
The state of California has mandated that licensed labs be able to detect category 1 pesticides at 100 ppb or less in any cannabis product. Furthermore, if a lab can detect category 1 pesticides at ANY level, the product fails. Therefore, if a lab is able to detect any category 1 pesticide at any level less than 100 ppb, the product fails for pesticides. This means that if lab A’s analytical method can detect 50 ppb but lab B’s method can detect only 100 ppb, a sample with 75 ppb of a category 1 pesticide will fail lab A’s test but pass lab B’s test, and both will be correct in the state’s eyes! For this reason it is important to understand the Limit of Detection (LOD) for pesticides for any lab you choose to work with as well as for any COA for raw material you may review. The state of California requires that a COA include these LODs for category 1 pesticides.
Pesticides analysis is sometimes referred to as residual pesticide analysis because these chemicals are found everywhere in the world after being used for decades on other crops. As such, these pesticides can be found in soil, water and other sources that may contaminant the plant during growth. Because cannabis has some of the most strict limits on pesticide content when compared to the food industry, infused product manufacturers may unintentionally adulterate their products in the manufacturing process by mixing THC/CBD oil with other ingredients that would not pass the state’s cannabis requirements for residual pesticides. For this reason, all infused product manufacturers should be testing every lot of every ingredient before mixing it into their formulation to ensure no contamination is passed into the final infused product.
When biomass is extracted to produce concentrates, pesticides are often carried with the cannabinoid oils such that the final product pesticide concentration is much higher than was in the original biomass. For example, if a biomass material with 9% THC concentration is concentrated, and the resulting oil is 90% THC, then the sample was concentrated 10 fold for THC and all many other analytes. Assuming all pesticides carried from the biomass into the final product just as THC did, they were also concentrated 10x. If pesticides were not visible in the starting biomass by a lab’s standard method because of their low concentration, the concentrate can still fail as the 10x increase in concentrations could bring the pesticide levels within the detection limit of the analytical method and above the regulated allowable limit set by state legislatures.
A solution to the above dilemma of using contaminated biomass to create concentrates is to modify the pesticide test method by first concentrating pesticides using a microextraction. This approach typically increases pesticides concentration by about 5 fold and is thus a good guide for selecting biomass with sufficiently low pesticide amounts to pass compliance testing of the final concentrate product. An alternative approach is to remediate the resulting concentrate, or distillate, if it’s taken one step further from the excess of pesticides. No universal method to solve this problem exists, but both extraction and chromatography have been used with some success.
Infused product manufacturing can begin with a cannabinoid concentrate that is completely free of heavy metal and pesticide contaminants, but the final product could fail compliance testing for containing these same analytes. This is possible because creating infused products includes mixing many other ingredients from other sources with cannabis concentrates to create the final product; therefore if one of the ingredients contains those contaminants, so will the final product, albeit at lower concentrations. For this reason, it is extremely important to test all ingredients for heavy metals and particularly pesticides before using them to create the final product or at least in large scale production if no test batch was created and tested first. Furthermore, it may not be sufficient to test ingredients from a vendor just once as there is lot to lot variability in those materials, so just because today’s ingredients are clean doesn’t mean that tomorrow’s will be.
Just because an ingredient does contain pesticides doesn’t mean that they are at levels above what is acceptable by state regulatory standards. If one of the ingredients does test positive, the impact on the final product’s pesticide concentration can easily be calculated. In order to do so, one just needs to know the total amount (mass) of the ingredient to be used in the batch and the combined size (mass) of the batch. For instance, if one is using 10 grams of sugar with 90 grams of other ingredients for a total of 100 grams for the batch, the contaminant will be diluted 10-fold in the mixing process. In other words, 10/100 = 10% of total batch mass comes from the contaminated ingredient, hence the final product’s contaminant concentration will also only be 10% of that in the ingredient.
Take an example where 10 g of orange peel essence that is contaminated with 10 ppm of imazalil, a category I pesticide, is mixed with 90 g of other recipe ingredients all completely void of imazalil. The final mixture therefore weighs 10x more than the 10-ppm-contaminated ingredient, and hence the concentration of imazalil in the mixture is decreased 10-fold to 1 ppm. As a category I pesticide, imazalil has a threshold at the LOD of the method (varies from lab to lab), with a minimum set at 0.1 ppm (0.1 ug/g), so in this scenario the final product will fail. Many food ingredients have much higher pesticide limits than cannabis products and many have no clear limits at all, so it is strongly recommended that all ingredients are tested for pesticides before use.
Contaminant concentrations levels will never increase when combining one ingredient with other ingredients unless the additional ingredients also contain the same pesticides. Under no circumstances will the combination of two contaminated ingredients create a mixture with even higher levels of that same contaminant.
The cannabis plant is a complex organism containing thousands of natural products, including hundreds of cannabinoids and terpenes, some of which are consumed by humans for recreational and medicinal purposes.. Analytical chemistry is an essential tool in the cultivation of cannabis and production of cannabis-derived products that enables people to understand the quantitative and qualitative properties of these products. Growing and processing cannabis may introduce contaminants such as pesticides, residual solvents and heavy metals that are regulated by state and federal agencies. Analytical testing labs are an essential component of the cannabis industry in order to regulate harmful chemicals. They are also essential for product developers and manufacturers in order to improve product quality and optimize manufacturing processes,
As an ISO/IEC 17025:2017 certified cannabis testing lab in Los Angeles, California, Pacific Star Labs empowers product developers and manufacturers with analytical chemistry expertise.
For Inquiries, Contact Us:
info@pacificstarlabs.com
Learn more about our services at pacificstarlabs.com
Download the Pacific Star Labs Cannabis Chemistry Guide to gain insights. A free resource to empower you.
-2.png?width=840&height=472&name=Cannabis%20Chemistry%20guide%20(1)-2.png)